Role
Dr Sophie Benjamin is a Senior Lecturer in Inorganic Chemistry and teaches on the chemistry course cluster (BSc, MChem, MRes and MSc). Her research interests encompass molecular main group and transition metal chemistry with a particular interest in co-ordination complexes of antimony and bismuth and unusual organometallic complexes. She likes to use as much of the periodic table as possible in both her research and her teaching. Dr Benjamin is also the Radiation Protection Officer for the University. She currently works part time (Mon-Thurs).
Career overview
After studying Natural Sciences at the University of Cambridge, Dr Benjamin completed her PhD in Inorganic Chemistry at the University of Southampton. Here she was then awarded an EPSRC Doctoral Prize Fellowship to undertake a postdoctoral research project. She moved to NTU to take up her current position in 2015.
Research areas
Research in the Benjamin group aims to synthesise and exploit new inorganic molecules, from the study of fundamental bonding to the design of new catalysts and antimicrobial agents, with a focus on the heavier elements of the p-block. For more information, visit the group website.
Transition metal complexes with heavy Group 15 ligands
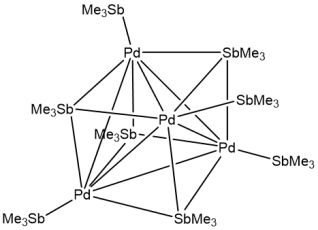

Bonds between heavy main group metals and transition metals come in many forms, and can strongly affect the properties of both metals. Because of the differences between heavy main group metals and more common, lighter donor atoms these complexes often have unusual structures and bonding types. One example is our beautiful [Pd4(SbMe3)8] cluster (J. Am. Chem. Soc., 2016, 138, 6964) which contains the first example of unsupported µ3-pnictine bonding (above).
Organometallic main group catalysts
Currently, most industrial catalysts are based on costly transition metals such as platinum, palladium, rhodium and gold, which have low natural abundance. While simple halides of main group elements have long been used as Lewis acid catalysts, these are generally corrosive and lack any tuneability. The development of catalysts based on organometallic main group derivatives is an attractive alternative for organic bond forming reactions. Main group metals such as antimony and bismuth are abundant and inexpensive (more than 20 times cheaper than platinum, for example), and the ability to vary the organic substituents gives scope to tailor the catalyst to the desired function. We are working to develop new organometallic main group catalysts and investigate their structure-function relationships.

Bismuth based antimicrobial agents
Our latest project aims to develop antimicrobial compounds based on the famously non-toxic metal bismuth. Bismuth complexes have antibacterial and antiviral properties, and in some cases can resensitise resistant bacteria to treatment with current antibiotics. However, only a few simple bismuth complexes have been used in these studies. We want to apply our expertise in bismuth chemistry to design and test new molecular bismuth complexes for use in antimicrobial applications, with the potential to control properties such as solubility, specificity and toxicity by synthetic modification. Watch this space!
Opportunities exist to conduct PhD and Masters level research in the group, please contact Dr. Benjamin direct.
External activity
Dr Benjamin is a member of the Royal Society of Chemistry and sits on the committee of the RSC coordination and organometallic discussion group (CODG) and the RSC main group discussion group.
Publications
Selected publications (see link below for full list):
Cationic Triarylchlorostibonium Lewis Acids. O. Coughlin, T. Krämer and S. L. Benjamin, Organometallics, 2023, 42, 339.
Diverse structure and reactivity of pentamethylcyclopentadienyl antimony(III) cations. O. Coughlin, T. Krämer and S. L. Benjamin, Dalton Trans., 2020, 49, 1726.
A Five-Membered PdSbn Coordination Series. A. Jolleys, B. R. M. Lake, T. Krämer and S. L. Benjamin, Organometallics, 2018, 37, 3854
[Pd4(μ3-SbMe3)4(SbMe3)4]: A Pd(0) tetrahedron with μ3-bridging trimethylantimony ligands. Benjamin, S.L., Krämer, T., Levason, W., Light, M.E., Macgregor, S.A. and Reid, G., 2016. Journal of the American Chemical Society, 138 (22), pp. 6964-6967.
Non-aqueous electrodeposition of functional semiconducting metal chalcogenides: Ge2Sb2Te5 phase change memory. Bartlett PN, Benjamin SL, (Kees) de Groot CH, Hector AL, Jolleys A, Huang R, Kissling GP, Levason W, Pearce SJ, Reid G* and Wang Y, Materials Horizons, 2015, 2, 420-426
Neutral Organoantimony(III) and Organobismuth(III) Ligands as Acceptors in Transition Metal Complexes – Role of Substituents. Benjamin SL, Reid G, Coordination Chemistry Reviews, 2015, 297298, 168-180
Chemical vapour deposition of antimony chalcogenides with positional and orientational control: precursor design and substrate selectivity. Benjamin SL, de Groot CH, Hector AL, Huang R, Koukharenko E, Levason W and Reid G, Journal of Materials Chemistry C, 2015, 3, 423-430
Bromostibine complexes of Fe(II): hypervalency and reactivity. Benjamin SL, Levason W, Light ME, Reid G and Rogers SM, Organometallics, 2014, 33, 2693
Controlling the nanostructure of bismuth telluride by selective chemical vapour deposition from a single source precursor. Benjamin SL, de Groot CH, Gurnani C, Hector AL, Huang R, Koukharenko E, Levason W and Reid G, Journal of Materials Chemistry, 2014, 2, 4865-4869 (Inside Cover)
Synthesis and reactions of a hybrid tristibine ligand. Benjamin SL, Levason W and Reid G, Organometallics, 2013, 32, 2760-2767
Medium and high oxidation state metal/non-metal fluoride and oxide-fluoride complexes with neutral donor ligands. Benjamin SL, Levason W and Reid G, Chemical Society Reviews, 2013, 42, 1460-1499
Halostibines SbMeX2 and SbMe2X: Lewis acids or Lewis bases? Benjamin SL, Levason W, Reid G and Warr RP, Organometallics, 2012, 31, 1025-1034
UN Sustainable Development Goals



